Abstract
Background. Primary immune thrombocytopenia (ITP) is an acquired form of thrombocytopenia caused by anti-platelet autoantibodies. The underlying mechanism is thought to involve the production of IgG autoantibodies specific for platelet membrane antigens such as glycoprotein (GP) IIb/IIIa and GPIb/IX, although the anti-platelet autoantibody testing is less sensitive. The ASH and the IWG guidelines for the management of ITP do not recommend routine testing of anti-platelet autoantibodies for the diagnosis of ITP, and thus diagnostic biomarkers for ITP remain to be developed. Although the principal pathophysiology of ITP is believed to be an IgG-mediated autoimmune disease, there are few evidence for the B-cell receptor (BCR) repertoires which are associated or related to this disorder.
Objectives. In order to identify the characteristics of IgG-BCRs that could be a potential candidate for a biomarker for primary ITP, we have investigated the comprehensive and precise repertoires of IgG-BCRs of peripheral blood B cells by using the next-generation BCR repertoire analysis comprised of high-throughput DNA sequencing and bioinformatics.
Materials and Methods. Eleven adult ITP patients and nine volunteer donors without any hematologic disorders were included in this study. Diagnostic criteria for ITP were based on the ASH and the IWG guidelines and secondary ITP was excluded. Written informed consent was obtained before drawing blood samples. Heparinized peripheral blood was taken and mononuclear cells were isolated by density gradient centrifugation. Total RNA extraction and cDNA synthesis were done by standard protocols. Unbiased amplifications of IgG-BCRs were performed by adaptor-ligation PCR and the amplicons were sequenced by next-generation sequencing. Each sequence read was analyzed by the bioinformatics software created by Repertoire Genesis Incorporation (Ibaraki, Japan), and the usage of IGHV, IGHD, IGHJ and IGHC and CDR3 sequences were determined, according to the previously reported (Iwamoto M, et al. ASH 2016, #4542). This study was approved by the institutional review board.
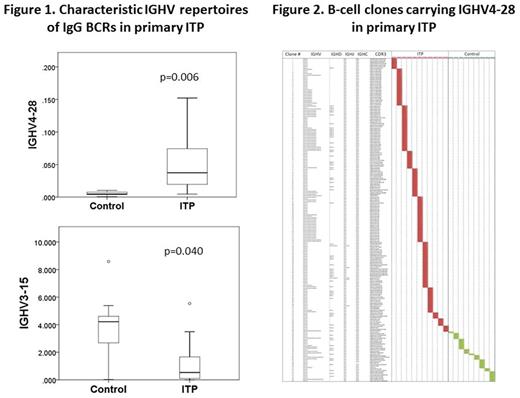
Results. Total 2,009,943 in-frame and 315,469 unique reads were obtained from twenty blood samples, and 29,049 to 160, 013 reads (100,497 reads in average) from each sample. Diversity of IgG BCRs was not different between the ITP patients and controls based on inverse Simpson and evenness Pielou's indexes. Comparisons of the IGHV repertoires covering fifty-nine IGHV subfamilies between the patients and controls revealed an increased usage of IGHV4-28 (0.053% vs. 0.005%, p=0.006) and a less usage of the IGHV3-15 (1.28% vs. 3.63%, p=0.04) segment in ITP patients (Fig. 1). The 220 distinct IGHV4-28-carrying sequences were identified in ITP patients and 135 out of these clones used the IGHJ4 gene segment (Fig. 2). These IGHV4-28/IGHJ4-carrying B-cell clones were found in all ITP patients. Only eight B-cell clones bearing IGHV4-28/IGHJ4 were identified in control donors. However, no identical IGHV4-28/IGHJ4-bearing B-cell clones were found across different ITP patients.
Discussion and Conclusion. The present study has demonstrated the preferential usage of the rearranged IGHV4-28/IGHJ4 gene by circulating IgG B cells in ITP. Roark et al. have previously reported the usage of IGHV3-30 by the anti-platelet immunoglobulin from two patients with ITP, demonstrated by the Fab/phage display libraries (Blood 2002;100:1388). Discordant results may be explained by the limited numbers of the patients examined or different HLA backgrounds. Although the antigen specificity of BCRs encoded by the IGHV4-28/IGHJ4 gene in the present patient cohort remains to be elucidated, our findings may contribute to the development of genetic testing for the diagnosis of ITP.
Kitaura: Repertoire Genesis Incorporation: Employment. Suzuki: Repertoire Genesis Incorporation: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal